How Many Moles Are in 22 G of Argon
Density of argon is equal to 17838 kgm³. 2 moles Argon to grams 79896 grams.
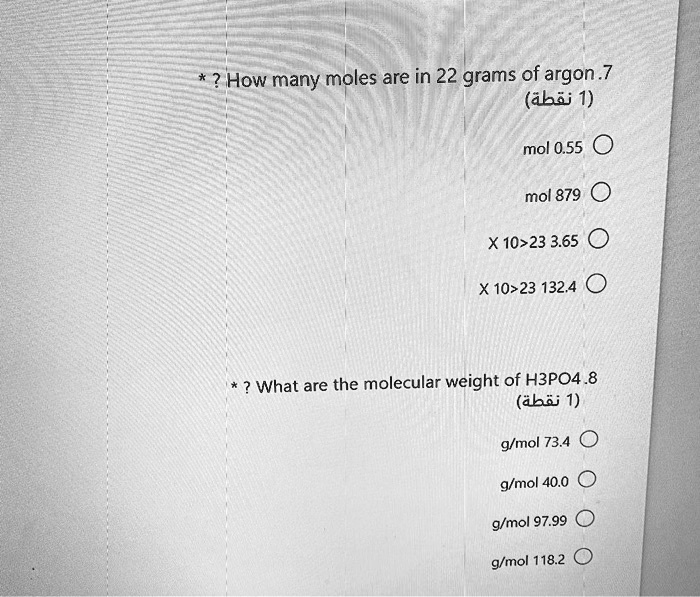
Solved How Many Moles Are In 22 Grams Of Argon 7 Abij 1 Mol 0 55 Mol 879 X10223 3 65 X10 23 132 4 What Are The Molecular Weight Of Hbpo4 8 Abi 1 Mmol 73 4 G Mol 40 0 G Mol 97 99 G Mol 118 2
Check out a sample QA here.

. 1 moles Argon to grams 39948 grams. Mathematically Number of moles Since it is given that mass is 22 grams and molar mass of argon is 40 gmol. Start by finding the molar mass of argon.
18 What is the mole of argon gas. A number of mole 22g 40 gmole 055 mole Ar. QuestionsToggle search form How many moles are grams Argon Posted April 2022 TSW Team The answer 39948.
Students whove seen this. 15 How many moles of argon are in 287 g of argon. 5 moles Argon to grams 19974 grams.
For example if argon has a mass of 22 grams and MgO has a mass of 881 grams then the atoms in argon have a molar masses of 60221023 molar mass of a molecule. Molar mass MgO 40 gmole. Convert the mass of each element to moles using the molar mass from the periodic table.
Secondly how many moles are in 22 grams of argon. Apure solvent has a vapor pressure the vapor pressure of a solution. Assume you are converting between grams Argon and mole.
14 How do you convert 20 grams of water to moles. At 0C 32F or 27315K at standard atmospheric pressureIn Imperial or US customary measurement system the density is equal to 011136 pound per cubic foot lbft³ or 00010311 ounce per cubic inch ozinch³. 22 grams of argon is equivalent to 055 moles.
B number of mole 881g 40 gmole 22 moles MgO. 12 How many atoms does ethanol have. Given argon Ar we know that the molar mass of arg.
Who are the experts. X22 40 055 moles of Argon. Molar mass Argon 40gmole.
4 moles Argon to grams 159792 grams. The molecullar mass for Argon is 40g mole. For argon the mass given 22 grams.
7 How many moles of carbon atoms and hydrogen atoms respectively are present in 3 moles of ethane. 9 How many atoms are in 05 moles of argon. Contents hide 1How many moles.
View the full answer. Also know how many moles are in Argon. The temperature of the gas in k not celsius.
Moreover how many moles are in 22 grams of argon answers. N 5988 g 18015 gmol 3324 mol. 17 What is a 1 mole.
How many moles are in 22 grams of argon. How many moles of argon are in 221 g of argon. Want to see the full answer.
Check out a sample QA here. Want to see the full answer. To get the number moles mass of the substance molar mass of the substance.
11 How many moles of carbon atoms are there in 3007 g of C2H6. Molecular weight of Argon or mol The molecular formula for Argon is Ar. Start with the number of grams of each element given in the problem.
Chemistry 21062019 1830 diawia. 6 moles Argon to grams 239688 grams. Mass given 881 g.
Beside above how many moles are in 452 grams of argon. 13 How many moles are in H2O. 3 moles Argon to grams 119844 grams.
As you already know how the grams to moles conversion work find the number of moles. 19 How do you find the mass number of argon. How many moles are in 25 grams of HF.
Therefore Number of moles 055 mol. How do I calculate moles. 8 How many atoms are in a mole of carbon.
Soobee72pl and 2 more users found this answer helpful. 7 moles Argon to grams 279636 grams. You can always use our grams to moles calculator to check the result.
Argon weighs 00017838 gram per cubic centimeter or 17838 kilogram per cubic meter ie. 12 How many molecules are there in 22 gram of carbon dioxide. 16 How many grams are in 24 moles of Sulphur.
Round to the nearest whole number. Use this page to learn how to convert between grams Argon and mole. Answer is 055 moles are in 22g of argon.
Thus we can conclude that there are 055 moles in 22 grams of argon. How many moles are in 22 grams of argon Other questions on the subject. How many moles are there in 22 grams of argon.
The number of moles is the substance of an element divided by its molar mass. Note that rounding errors may occur so always check the results. 8 moles Argon to grams 319584 grams.
1 grams Argon is equal to 0025032542304996 mole. Divide each mole value by the smallest number of moles calculated. Experts are tested by Chegg as specialists in their subject area.
10 How many atoms of argon are there in 0500 moles AR. 4How many moles are there in 74 x 1023 molecules of AgNO3. Using this equation we can take 2510 19 and find that it is equal to 125 moles.
We review their content and use your feedback to keep the quality high. 3 How many moles are in 22 grams of argon. The SI base unit for amount of substance is the mole.
5How Get the answers you need now.

How Many Moles Are In 22 Grams Of Argon Healing Picks
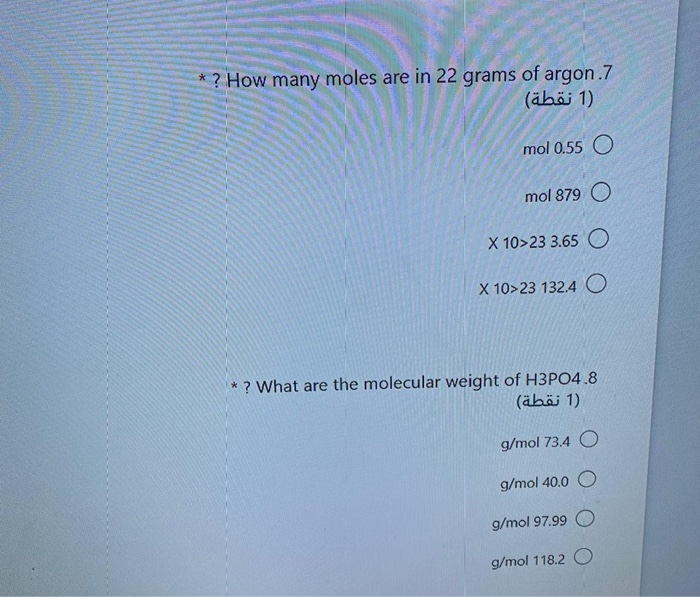
Solved How Many Moles Are In 22 Grams Of Argon 7 1 1 Chegg Com

Mole Conversions Worksheets Answer Key Mole Conversion Worksheet Mole Conversion Chemistry Worksheets
Comments
Post a Comment